Abstract
Background: Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). While many factors influence the outcomes of allo-HCT in these leukemias, the independent impact of ABO mismatching between the patient and donor remains unclear.
Methods: Using the CIBMTR database, we identified adults aged ≥18 years with AML or ALL who underwent allo-HCT from HLA-matched sibling donor (MSD) or 8/8 matched unrelated donor (MUD) between 2008-2018. We excluded patients who underwent transplant from a mismatched donor source or who received an ex-vivo T-cell depleted graft. Patients were stratified into cohorts based on ABO status (match, minor mismatch, major mismatch, bidirectional mismatch). Outcomes such as overall survival (OS), disease free survival (DFS), non-relapse mortality (NRM), relapse, incidence of acute graft versus host disease (GVHD), chronic GVHD, neutrophil engraftment, platelet engraftment and graft failure were evaluated. Survival analysis was done using Kaplan-Meier method and significant predictors were evaluated using Cox-proportional hazard regression method. Multivariate regression model included main effect (ABO status) and covariates (patient age, gender match, disease type, disease status, HCT-CI, Karnofsky performance status, donor type, conditioning intensity/use of TBI, stem cell source, GVHD prophylaxis, ATG/alemtuzumab use, transplant year).
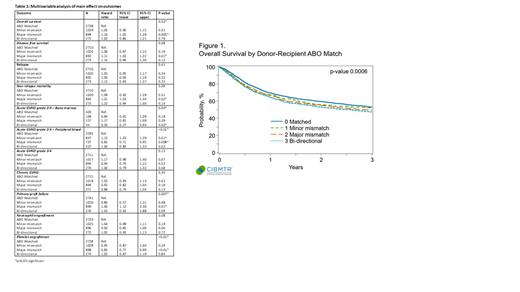
Results: Of 4946 patients who met the study criteria, 2741 patients (55.4%) were ABO matched, 1030 patients (20.8%) had minor ABO mismatch, 899 patients (18.1%) had major ABO mismatch and 276 patients (5.6%) had bidirectional ABO mismatch. Graft manipulation for ABO incompatibility was performed in 900 patients (minor ABO mismatch=532; major ABO mismatch=226; bidirectional mismatch=142), however, the information on individual graft manipulation techniques was limited. In multivariable analysis (table 1), major ABO mismatch significantly affected the OS, platelet engraftment and primary graft failure. Compared to ABO matched allo-HCT, major ABO mismatched allo-HCT was associated with worse OS (major mismatch - HR 1.16, 95% CI 1.05-1.29; p=0.005), inferior platelet engraftment (HR 0.83, 95% CI 0.77-0.90; p=<0.001), and higher risk of primary graft failure [4.5% (major mismatch) vs. 3.2% (ABO matched) - HR 1.60, 95% CI 1.12-2.30, p=0.01]. There was a significant interaction between the ABO status and graft type (peripheral blood vs. bone marrow) for the acute GVHD grades 2-4 model, and they are presented separately (table 1). Bidirectional ABO mismatch also significantly impacted the outcomes such as acute GVHD grades 2-4 (in bone marrow stem cell subgroup, HR 0.50, 95%CI 0.27-0.93, p=0.02) in addition to a trend towards inferior survival and NRM (p-value not significant). Other outcomes such as relapse (p=0.41), acute GVHD grades 3-4 (p=0.13), and chronic GVHD (p=0.30) were not significantly influenced by the ABO status.
Conclusions: Our study demonstrates that pre-transplant ABO status is an independent predictor of survival and other post-transplant outcomes in a large cohort of patients with AML and ALL undergoing allo-HCT in the recent era. This demonstrates the importance of considering ABO status in the donor selection algorithms and potential strategies to mitigate its adverse impact. Due to the limited information available on graft manipulation strategies, the impact of graft manipulation techniques on the outcomes could not be evaluated and needs to be investigated in future studies.
Guru Murthy: Techspert: Consultancy; Guidepoint: Consultancy; Cancerexpertnow: Honoraria; Qessential: Consultancy; TG therapeutics: Other: Advisory board; Cardinal Health Inc.: Honoraria. Devine: Magenta Therapeutics: Current Employment, Research Funding; Tmunity: Current Employment, Research Funding; Sanofi: Consultancy, Research Funding; Johnsonand Johnson: Consultancy, Research Funding; Orca Bio: Consultancy, Research Funding; Be the Match: Current Employment; Vor Bio: Research Funding; Kiadis: Consultancy, Research Funding. Farhadfar: Incyte: Consultancy. Sharma: Vertex Pharmaceuticals/CRISPR Therapeutics: Other: Salary support paid to institution; Novartis: Other: Salary support paid to institution; Spotlight Therapeutics: Consultancy; Medexus Inc: Consultancy; CRISPR Therapeutics: Other, Research Funding; Vindico Medical Education: Honoraria. Stefanski: Novartis: Honoraria. Pulsipher: Equillium: Membership on an entity's Board of Directors or advisory committees; Adaptive: Research Funding; Jasper Therapeutics: Honoraria. Shaw: mallinkrodt: Other: payments; Orca bio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal